STEPS TO STUDY ACTIVATION
Complete the Departmental Financial Setup
A key step in activating a clinical research study is confirming that a study account is active and that the necessary purchasing and invoicing processes are established to facilitate and process study-related payments. In addition to the guidance below, investigators should contact their department finance or business managers to ensure that unit specific procedures are followed.
Affiliate (IU Health Teams) Differences in Process
IU Health teams will not have research accounts. For Advarra Payments, Financial Reference Number= n/a and select “IU Health-ECRO” as the business unit.
For Indiana University departments, all sponsored research accounts, regardless of sponsor type, are established by the Office for Research Administration (ORA). If a study lacks external funding, an internal department account should be designated to cover trial-related costs. A study account number will be required when completing other steps in the clinical research roadmap. ORA typically creates accounts once a contract or award is finalized, although study teams can request an advanced account for clinical research agreements.
Advanced Accounts
An advanced account enables you to charge specific non-patient-related expenses to your pending industry-sponsored contract, avoiding the need for later adjustments. Additionally, an advanced account facilitates progression with other study activation steps before finalizing the contract. To request an advanced account, the principal investigator, project director, or fiscal officer must complete an Advanced Account Document in Kuali.
To learn more about Advanced Accounts and access the Advanced Account Request document, visit the ORA Advanced Accounts website. (Note: CAS Authentication and special permissions are required.) You may also wish to review the university policy on Requests for Advanced Accounts.
If you have questions related to sponsored research accounts, please contact ORA at ora@iu.edu.
After setting up the study account, the team should evaluate the need for study-related purchase orders and complete the necessary requisitions in BUY.IU, IU’s procurement system. A IU purchase order is necessary for any payment to an external entity related to the study. To access BUY.IU visit One.IU and Search for “BUY.IU.” (Note: CAS Authentication and special permissions are required.)
Common clinical research purchase orders created by IU study teams include payments to IU Health for study services. When creating a requisition in BUY.IU, ensure the correct remittance and fulfillment address is used. Refer to this document to identify the appropriate addresses for each of the common IU Health research service billing departments.
*Device Purchase Orders* Please note that purchase orders for acquiring study devices or supplies MUST be made through IU Health Purchasing if the device/supply will be billed by IU Health to the research study or to the patient as a routine cost.
For information about BUY.IU training and access, please visit the Financial Training & Communications Purchasing website.
Advarra OnCore Financials is a central platform for clinical trial financial management. It streamlines budgeting for study events like consents, enrollments, and visits, automates invoice generation, and tracks payments. Benefits include reduced manual data entry, enhanced regulatory compliance, real-time budget insights, and better communication between research and financial teams, leading to time savings, fewer errors, and optimized workflows.
Use of the OnCore Financials Console is optional at this time. Study teams opting to use this feature must complete OnCore Financials setup before completing the Research Unit Signoff in the OnCore Protocol status workflow.
For more information about using OnCore Financials for your study, please contact the OCR Research Systems team at oncore@iu.edu.
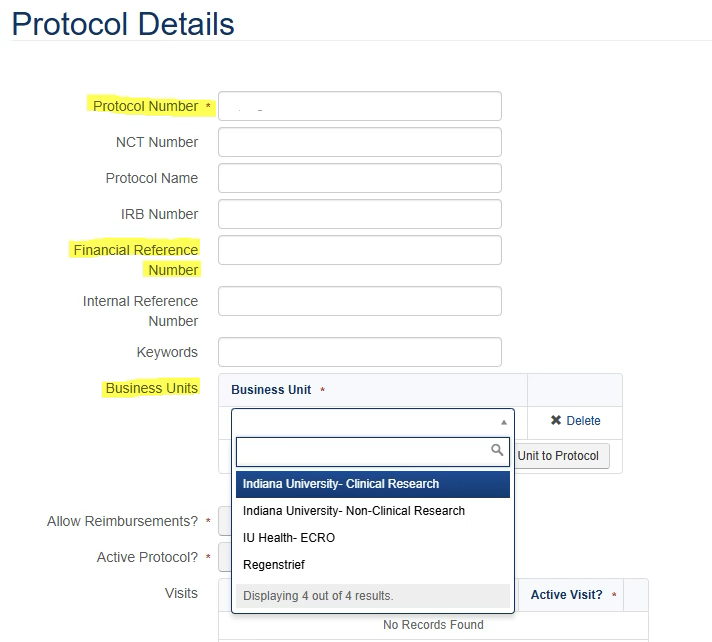
If you will be using the Advarra Participant Payments system to pay your research participants. Your study account number must be placed in the Financial Reference Number field in the Payments Protocol Details Screen. If applicable, the sub-account number should be placed in the Internal Reference Number field. Do not forget to select your Business Unit.
More information on the Advarra Payments system is available in the Department Setup of Participant Payments tile.
For Cancer Center Trials (studies involving cancer patients):
- For studies involving the Cancer Center’s Adult Clinical Trials Office (CTO), please reach out to cto-finance-l@list.iu.edu for more specific details on study financial setup.
- For studies involving the Cancer Center’s Pediatric Team, please reach out to rileyphosresearchteam@iuhealth.org for support with study financial setup.
Completing the department financial setup is only one component of study activation. Additional requirements should be reviewed using the Clinical Research Roadmap.