STEPS TO STUDY ACTIVATION
Conduct a Feasibility Review
Before a study can be performed, researchers and/or research teams should conduct a review for feasibility. This means answering the question, “Can this study be done successfully at our site(s)?” Often, this will consist of gathering data about the resources available at the institution, which helps determine the parameters of the study and whether the study will be viable.
Feasibility Review Types
There are two types of feasibility review, and each has their own questions that need to be answered.
Sponsor Feasibility Questionnaires are a set of questions prepared by a study sponsor or contract research organization (CRO) to identify a potential site and gauge the interest of a site/investigator to run a particular clinical trial successfully. There is no widely accepted or standardized approach to the questionnaire, so each of them is different and will contain various questions based on what the Sponsor is looking for. Examples of questions that may be asked during a Sponsor Feasibility Review include:
- How many patients of this population are seen at the site each year? Diversity breakdown of these patients?
- Based on inclusion/exclusion criteria, how many subjects can the site enroll?
- What research experience does the PI and research team have?
- Can required procedures/testing be completed at the site?
- Are there any other committees or approvals that must be obtained besides IRB approval?
- Expected timelines for milestones such as contract and budget execution, IRB submission, and total time from start up to open to enrollment
- Does the site have the appropriate space and equipment to carry out the study?
An internal feasibility review is a set of questions prepared by departments within the IU and IU Health organization. These questions are meant to help study teams assess a particular study’s needs and ensure success in operationalizing the trial and meeting commitments to the sponsor. Questions that are explored may vary from department to department. This assessment will occur after sponsor feasibility review. Investigators and their teams should take the time to review the study protocol and other pertinent documents such as pharmacy manual, lab manual, etc. to ensure they can be successful in the study. Examples of questions that one should ask during an Internal Feasibility Review include:
- Can the type of research the study is designed for be conducted at the site?
- Does the site have the patient population to conduct the study?
- Are there competing trials?
- Does the PI have support collaborators (multiple modalities)
- Does the expected enrollment goal seem achievable based on inclusion/exclusion criteria?
- Do team members have the proper resources, tools, training, and time to devote to the study?
- Does IDS have the capacity to accept the study responsibilities?
- Do other departments (clinics, inpatient units, Clinical & Translational Science Laboratory, Cancer Center Cores, Nuclear Medicine, Cellular Therapy Facilities, Apheresis facilities, etc.) that might be utilized, staff or space, have the capacity to accommodate the study responsibilities and/or increase in patient population?
- Can the PI provide adequate oversight (ex. current study load, dedicated time for research, etc.) ?
- Is the cost of the study offset by the returns of the study?
- Does the site have all the equipment needed for the study (exam room space, freezers, centrifuge, EKG machine etc.)
- Will the site need help from other teams within the IU/IUH system to do the study (CTSL, CRC, ECRO, etc.) ?
INTERNAL FEASIBILITY REVIEW: Recruitment Decision Flow Diagram
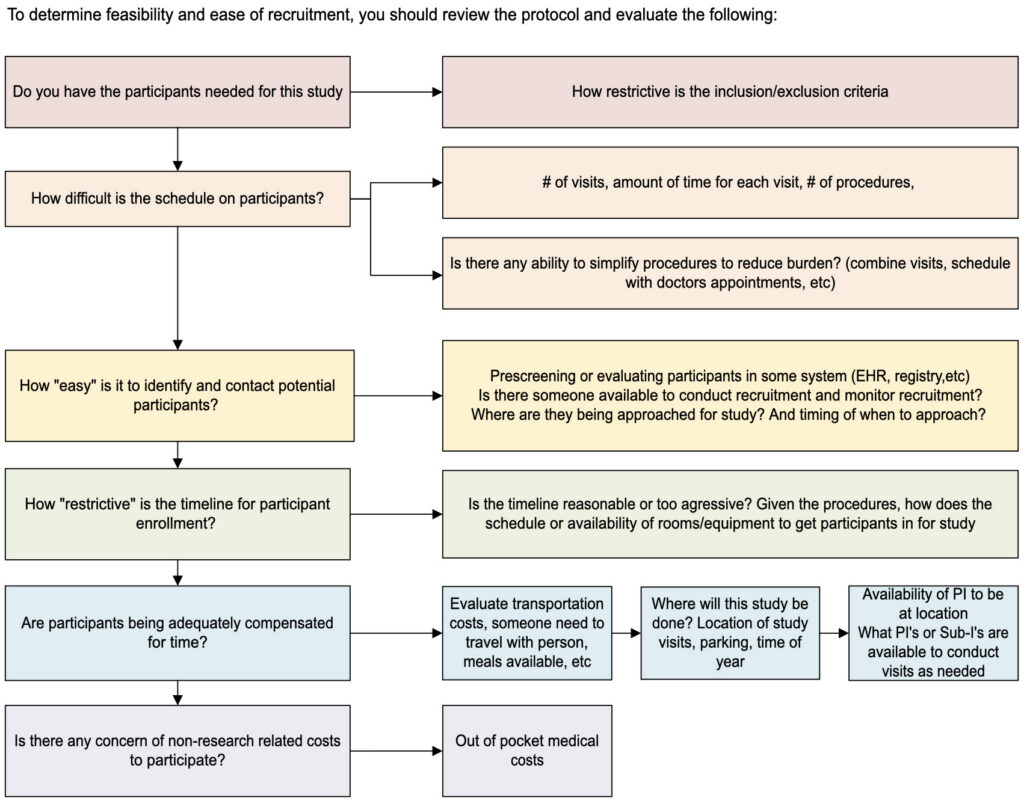
Patient Population Statistics
In many cases, having a report that details specific criteria of interest in a study will help determine whether that study will be viable. For example, reports can be run on the number of patients who meet the disease or condition of interest to gauge whether there is sufficient population available and to estimate the number of patients who could be enrolled.
Groups to turn to in these cases could be the Regenstrief Institute, IU Health’s data brokerage, or the IU Health Clinical Research Systems. Depending on the study, the Clinical and Translational Sciences Institute of Indiana (CTSI) may also be appropriate. To see which group(s) may be right for assistance in your study’s Feasibility Review, refer to the list of contacts or resources below.
Your study may require feasibility reviews through various entities throughout the IU Health and IU collaborative network. To see if you need additional feasibility reviews, please browse the list below. Here, you will find links to specific resources and contacts to help determine whether you will need to submit to a particular review.
IU Simon Comprehensive Cancer Center (IUSCCC) Additional Information
If your study involves resources, patients, or space within the IUSCCC, there may be additional Feasibility Review item to consider. For the best, most up-to-date information, please refer to the IUSCC’s webpage (needs an IU login) or contact the Clinical Trials Office at CROSRC@iu.edu.
The IUSCCC has a feasibility form that is completed through REDCap. Please reach out to the contact above for more information.
IU Affiliates (IU Health Teams) Additional Information
If your studies will involve the IU Health facilities, please reach out to ISClinicalResearchSupport@iuhealth.org.
Contacts and Resources
Regenstrief Institute
- Serves as electronic medical record data warehouse for IU Health and Eskenazi
- Central point of access to date from Indiana Network for Patient Care
- Can provide aggregate data based on inclusion/exclusion criteria
- For more information or to submit a request, please contact: askRDS@regenstrief.org
IU Health Clinical Research Systems
- Provides feasibility numbers based on discreet data fields such as IDC-10 codes, lab values, location, etc.
- Reaches across all IU Health facilities including both inpatient and outpatient encounters
- For more information, please contact: ISClinicalResearchSupport@iuhealth.org
Completing the Feasibility Review is only one component of study activation. Additional requirements should be reviewed using the Clinical Research Roadmap.
